
Dr. Scott Weinert
Office: 319 Physical Science I
Phone: (405) 744-6543
charles.s.weinert@okstate.edu
Latest News
-
Vanessa Successfully Defends Her PhD Thesis!
On July 10th 2025, Vanessa Fortney sucessfully defended her PhD thesis. She came to OSU with very...
-
Laura delivers a poster at the 2025 CSC in Ottawa
Laura Levescy presented her research at the poster session at the 2025 Canadian Society for...
-
Vanessa presents her reserach at the CSC 2025 in Ottawa
Vanessa presents her research at CSC 2025 in Ottawa Vanessa gave an oral presenation of her latest...
-
Weinert group attends the Spring 2025 MICA Meeting
Vanessa, Laura, and Scott attended the Spring 2025 Midsouth Inorganic Chemists Association (MICA)...
-
Vannessa presents her latest results at CSC Winnipeg
Vanessa Fortney gave a talk during the General Inorganic Session at the Canadian Soicety of...
Research
Research in the Weinert group is primarily focused on the synthesis and characterization of oligogermanes, which are the germanium analogues of hydrocarbons that contain germanium – germanium single bonds. The compounds are of interest due to their inherent σ-delocalization, where the electrons in the highest occupied molecular orbitals of these compounds are not localized between two individual atoms but rather are typically delocalized across the entire germanium – germanium framework. This occurs when the germanium atoms are disposed in a trans-coplanar orientation that then allows the overlap of the diffuse 4sp3 orbitals to occur (Figure 1).
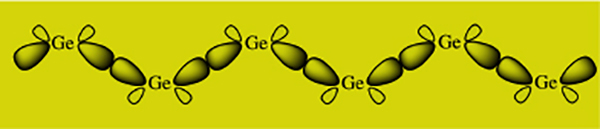
FIGURE 1
The construction of these molecules is not trivial and previously reported synthetic methods were replete with complications that did not allow for a detailed study of these systems. We developed the hydrogermolysis reaction in 2006 for the synthesis of these molecules, and this serves as a useful tool for the construction of a wide variety of systems (Scheme 1). Using this method we have prepared numerous new oligogermanes having a variety of chain lengths and substituent patterns, and this has included linear, branched, and cyclic systems.
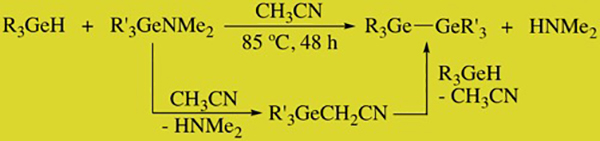
SCHEME 1
The ultimate goal of this work is to prepare molecules that have useful optical, electronic, or conductive properties. Such physical attributes have been seen in polygermanes, which are systems with long chains and a dispersion of molecular weights, or germanium nanomaterials. We are attempting to prepare fully characterized small molecules that exhibt such properties. To this end, we reported the hexagermane Pri3Ge(GePh2)GePri3 and have studied its properties in detail (Scheme 2, Figure 2). This molecule is thermochromic and also luminescent in both solution and in the solid state, and we postulate that the luminescence is due to a conformational change in the solid state upon excitation. There is an intense blue emission in the soid state at 440 nm at 80 K when the molecule is excited at 300 nm, and this can be visually observed as well (Figure 3).
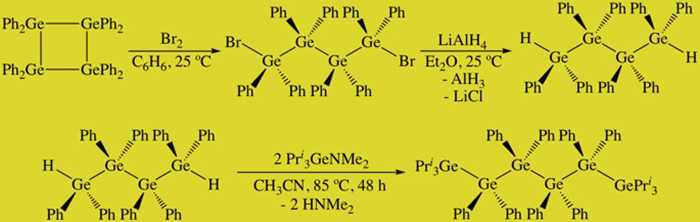
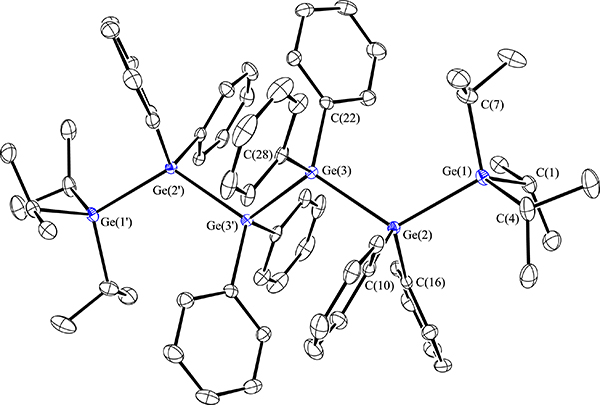
FIGURE 2
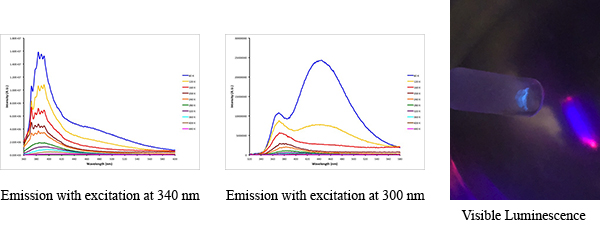
FIGURE 3




